Superior methanol electrooxidation activity and CO tolerance of mesoporous helical nanospindle-like CeO2 modified Pt/C†
Abstract
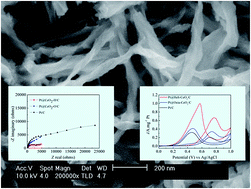
In an attempt to enhance the electrocatalytic activity and CO tolerance of ceria modified Pt/C electrodes, a novel structured ceria material has been developed. Left-handed helical CeO2 nano-spindles with mesoporous structures were successfully synthesized through a template-free based precursor method on a large scale. By a microwave-assisted polyol synthesis process, the ceria modified Pt/C electrocatalysts were synthesized. The helical CeO2 nanospindle based electrode Pt@Heli-CeO2/C exhibits superior electrochemically active surface areas, and significantly enhanced methanol oxidation catalytic activity and CO antipoisoning activity, compared to Pt/C and a nano-octahedral CeO2 modified electrode Pt@Octa-CeO2/C. The experimental results show that Pt@Heli-CeO2/C possesses 8.2 times and 3.2 times higher activity for methanol electrooxidation than Pt/C and Pt@Octa-CeO2/C, respectively. This remarkable enhancement could be attributed to the reasons as follows: compared to octahedral CeO2, the unique helical CeO2 is more conductive with better electron transfer and can provide more active surface sites to strengthen the support–metal interactions based on an electronic transfer mechanism from CeO2 to Pt, thus helical CeO2 can promote better dispersion of the Pt(0) nanocrystallites and a high concentration of metallic Pt(0) in the composition.

 Please wait while we load your content...
Please wait while we load your content...