Acylation or phosphorylation of hydroxyurea unexpectedly takes place on N rather than on O, leading to the formation of amides instead of the expected esters†
Abstract
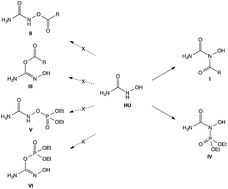
Attempted acylation of the anticancer agent hydroxyurea (HU) with acyl chlorides or anhydrides led to acylation on the NH group rather than on the OH. The structures of the products were confirmed by 15N-HMBC NMR. An analogous reaction conducted with hydroxamic acids (RCONHOH) or N-hydroxycarbamates (ROCONHOH) led to acylation on the OH. Surprisingly, despite the established affinity of phosphorous to O, phosphorylation of HU also took place at the NH group instead of the OH. These results are rationalized based on the different dominant resonance structures of HU, the hydroxamic acids or the N-hydroxycarbamates.

 Please wait while we load your content...
Please wait while we load your content...