A new comonomer design for enhancing the pH-triggered LCST shift of thermosensitive polymers†
Abstract
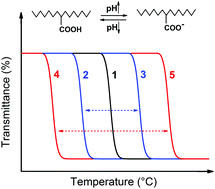
A thermosensitive polymer exhibiting a lower critical solution temperature (LCST) can be made responsive to pH change by introducing acid or base comonomer units, and the LCST can be switched between higher and lower temperatures as a result of the polarity change of the comonomer units upon their pH-induced protonation or deprotonation. In the present study, we describe a new comonomer design that aims at increasing the magnitude of the pH-triggered LCST shift. Random copolymers of N-isopropylacrylamide and 4-((2-carboxyallyl)oxy)benzoic acid, denoted as P(NIPAM-co-CBA), were synthesized, in which each CBA comonomer unit bears an acrylic acid and a benzoic acid group of similar pKa. With respect to comonomers containing a single acid group, this particular comonomer structure makes it more hydrophobic in the protonated state (pH < pKa) due to the phenyl group and more hydrophilic in the deprotonated state (pH > pKa) due to the double charge, which results in a larger pH-triggered LCST shift based on the comonomer effect. The demonstrated comonomer design principle, which is general and can also be applied to base comonomers, represents a useful strategy for enhancing the efficiency and sensitivity of the pH-responsiveness of LCST polymers.

 Please wait while we load your content...
Please wait while we load your content...