Precision synthesis of macrocyclic giant surfactants tethered with two different polyhedral oligomeric silsesquioxanes at distinct ring locations via four consecutive “click” reactions†
Abstract
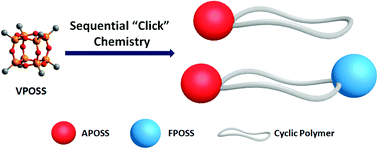
The combined utilization of chemoselective “click” chemistry allows for the preparation of well-defined macromolecules with complex compositions and architectures. In this article, we employed the sequential “click” strategy to further expand the scope of synthetically available giant molecules by precisely constructing new giant surfactants based on polyhedral oligomeric silsesquioxane (POSS) tethered cyclic polymers. The general synthetic approach involves sequentially performed strain-promoted azide–alkyne cycloaddition (SPAAC) as a method for bimolecular homobifunctional ring closure, copper-catalyzed azide–alkyne cycloaddition (CuAAC) for POSS-polymer conjugation, and thiol–Michael/thiol–ene reactions for POSS surface functionalization. Specifically, a cyclic polymer tethered with two POSS cages of distinct surface chemistry at different locations of the chain has been prepared. This work promises to afford numerous cyclic polymers-based giant surfactants with diverse structural variations for further investigation on unexpected physical properties.

 Please wait while we load your content...
Please wait while we load your content...