Donor–acceptor block copolymers carrying pendant PC71BM fullerenes with an ordered nanoscale morphology†
Abstract
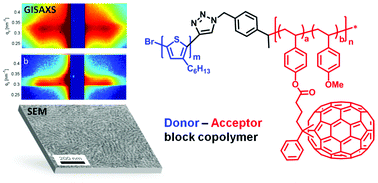
We present a straightforward method for the preparation of a novel donor–acceptor block copolymer based on an acceptor block with pendant phenyl-C71-butyric methyl ester (PC71BM) and a regioregular poly(3-hexylthiophene) (P3HT) as a donor. First, a hydroxyl-functionalized polystyrene copolymer with an azide end group was synthesized via nitroxide-mediated radical polymerization (NMRP) and was coupled with alkyne-terminated P3HT using copper(I) catalyzed azide–alkyne cycloaddition (CuAAC). The grafting reaction of phenyl-C71-butyric acid (PC71BA) to the hydroxyl groups of the polystyrene precursor was optimized to yield near-quantitative conversion which is demonstrated for a PC71BM-grafted acceptor copolymer in detail using MALDI-TOF mass spectrometry, thermogravimetric analysis (TGA) and 1H-NMR spectroscopy. Owing to the incorporation of C70, the donor–acceptor block copolymer exhibits enhanced absorption in the entire visible range of 300 to 600 nm. A detailed structural analysis of the block copolymer based on small-angle X-ray scattering in transmission (SAXS) and in grazing incidence geometry (GISAXS) as well as scanning electron microscopy (SEM) gave clear evidence for the formation of a periodic nanostructure of 37 nm in bulk and in thin films.

 Please wait while we load your content...
Please wait while we load your content...