Efficient access to conjugated 4,4′-bipyridinium oligomers using the Zincke reaction: synthesis, spectroscopic and electrochemical properties†
Abstract
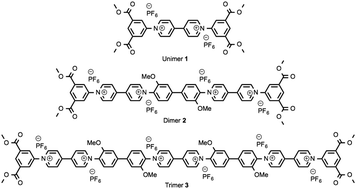
The cyclocondensation reaction between rigid, electron-rich aromatic diamines and 1,1′-bis(2,4-dinitrophenyl)-4,4′-bipyridinium (Zincke) salts has been harnessed to produce a series of conjugated oligomers containing up to twelve aromatic/heterocyclic residues. These oligomers exhibit discrete, multiple redox processes accompanied by dramatic changes in electronic absorption spectra.


 Please wait while we load your content...
Please wait while we load your content...