Total syntheses of natural products containing spirocarbocycles
Abstract
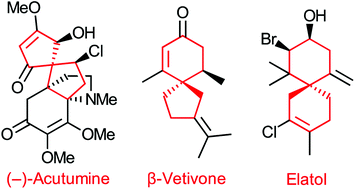
The structures of natural products from a variety of sources contain spirocycles, two rings that share a common atom. The spiro motif is finding increasing inclusion in drug candidates, and as a structural component in several promising classes of chiral ligands used in asymmetric synthesis. Total syntheses of products containing all-carbon spirocycles feature several common methods of ring closure which we examine in this review.

 Please wait while we load your content...
Please wait while we load your content...