Dual-mode chemodosimetric response of dibromo-BODIPY with anions†
Abstract
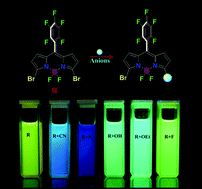
Aromatic nucleophilic substitution (Ar-SN) reaction of 3,5-dibromopentafluorophenyl-BODIPY has been explored as a remarkable basis for selective discrimination of anions. The efficient and characteristic ability of anions to modulate the absorption and emission properties of the dye provides an instantaneous distinction through dual-modes. For the first time, a novel platform to achieve dual-modal and promising recognition with discrimination of a series of anions differing in the nucleophilic atoms (F, O, C and N) has been taken into consideration. The behaviour of various anions with dibromo-BODIPY and vivid signal transduction has been fully established with absorption and emission spectroscopy. In addition to this, recognition events have been unambiguously characterized by 1H, 19F-NMR and single-crystal XRD.

 Please wait while we load your content...
Please wait while we load your content...