A study of the norcaradiene–cycloheptatriene equilibrium in a series of azulenones by NMR spectroscopy; the impact of substitution on the position of equilibrium
Abstract
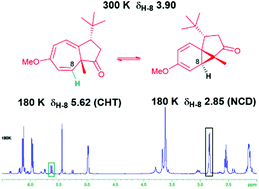
A systematic investigation of the influence of substitution at positions C-2 and C-3 on the azulenone skeleton, based on NMR characterisation, is discussed with particular focus on the impact of the steric and electronic characteristics of substituents on the position of the norcaradiene–cycloheptatriene (NCD–CHT) equilibrium. Variable temperature (VT) NMR studies, undertaken to enable the resolution of signals for the equilibrating valence tautomers revealed, in addition, interesting shifts in the equilibrium.

 Please wait while we load your content...
Please wait while we load your content...