Understanding the conformational behaviour of Ac-Ala-NHMe in different media. A joint NMR and DFT study†
Abstract
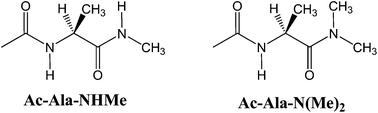
The conformational behaviour of Ac-Ala-NHMe was studied in the gas-phase and in solution by theoretical calculations (B3LYP-D3/aug-cc-pVDZ level) and experimental 1H NMR. The conformational preferences of this compound were shown to result from a complex interplay between the strengths of possible intramolecular hydrogen bonds, steric interactions, hyperconjugation, entropy effects and the overall dipole moments. The Ac-Ala-N(Me)2 derivative was studied in addition, to design a system akin to Ac-Ala-NHMe, but with disrupted intramolecular hydrogen bonds involving the -NHMe group, mimicking the effect of polar protic solvents.

 Please wait while we load your content...
Please wait while we load your content...