A facile and regioselective synthesis of 1-tetralones via silver-catalyzed ring expansion†
Abstract
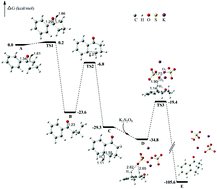
A regioselective synthesis of 1-tetralones via silver-catalyzed ring expansion is described. A variety of 1-tetralones are furnished under mild reaction conditions from tertiary cyclobutanols regardless of the electronic properties and steric hindrance of substituents, providing a new and practical method to access diverse 1-tetralone building blocks. Preliminary experimental and DFT studies revealed that a radical-mediated sequence of C–C bond cleavage/C–C bond formation is involved.

 Please wait while we load your content...
Please wait while we load your content...