Application of design of experiments (DoE) optimization to the one-pot synthesis of 4,6-dihydropteridinones†
Abstract
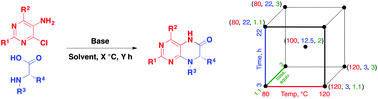
A design of experiments (DoE) analysis of a tandem SnAr-amidation cyclization reaction between 4-chloropyrimidin-5-amine and (S)-N-methylalanine to form (S)-7,8-dimethyl-7,8-dihydropteridin-6(5H)-one is reported. Five reaction variables were optimized using DoE and conversion was improved from 26% to 74%, with a significant reduction in reaction time while retaining high optical purity. The optimized conditions were applied to the synthesis of a wide variety of analogs and the expanded reaction substrate scope included a variety of amino acids and pyrimidines. Products were obtained in isolated yields up to 95% and enantiomeric excess as high as 98%.

 Please wait while we load your content...
Please wait while we load your content...