Synthesis of virtually enantiopure aminodiols with three adjacent stereogenic centers by epoxidation and ring-opening†
Abstract
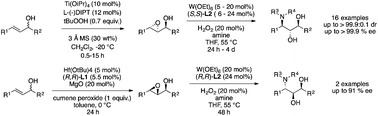
A virtually complete enantioselective synthesis of 3-amino-1,2-diols with three consecutive stereocenters was accomplished by a sequential cascade of two kinetic resolutions, which features a Sharpless or Hafnium-catalyzed asymmetric epoxidation and a subsequent W-catalyzed aminolysis. Enantiopure products with up to >99.9% ee and >99.9 : 0.1 dr were obtained and could serve as potential building blocks for pharmaceutical or biological significant molecules.

 Please wait while we load your content...
Please wait while we load your content...