Total synthesis of two potent anti-inflammatory macrolactones of the oxacyclododecindione type†
Abstract
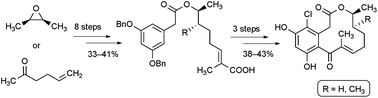
An esterification/Friedel–Crafts-cyclization approach permitted the first successful synthetic entry into the oxacyclododecindione subclass of the dihydroxyphenylacetic acid lactone-type natural products. This route allowed the preparation of two highly active anti-inflammatory fungal secondary metabolites 14-deoxyoxacyclododecindione and 14-deoxy-4-dechlorooxacyclododecindione as well as their 14-desmethyl analogues.

 Please wait while we load your content...
Please wait while we load your content...