An investigation of nitrile transforming enzymes in the chemo-enzymatic synthesis of the taxol sidechain†
Abstract
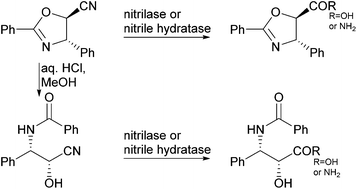
Paclitaxel (taxol) is an antimicrotubule agent widely used in the treatment of cancer. Taxol is prepared in a semisynthetic route by coupling the N-benzoyl-(2R,3S)-3-phenylisoserine sidechain to the baccatin III core structure. Precursors of the taxol sidechain have previously been prepared in chemoenzymatic approaches using acylases, lipases, and reductases, mostly featuring the enantioselective, enzymatic step early in the reaction pathway. Here, nitrile hydrolysing enzymes, namely nitrile hydratases and nitrilases, are investigated for the enzymatic hydrolysis of two different sidechain precursors. Both sidechain precursors, an openchain α-hydroxy-β-amino nitrile and a cyanodihydrooxazole, are suitable for coupling to baccatin III directly after the enzymatic step. An extensive set of nitrilases and nitrile hydratases was screened towards their activity and selectivity in the hydrolysis of two taxol sidechain precursors and their epimers. A number of nitrilases and nitrile hydratases converted both sidechain precursors and their epimers.

 Please wait while we load your content...
Please wait while we load your content...