A divergent approach to benzylisoquinoline-type and oxoaporphine alkaloids via regioselective direct ring metalation of alkoxy isoquinolines†
Abstract
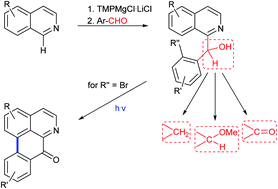
Methoxy- and benzyloxy-substituted isoquinolines are regioselectively metalated at C-1 with the Knochel–Hauser base, subsequent trapping with aromatic aldehydes gives aryl(isoquinolin-1-yl)carbinols as building blocks for divergent syntheses of different types of benzylisoquinoline alkaloids. Photochemical cyclization of ortho-bromo analogues under reductive conditions gives oxoaporphine alkaloids. Nine benzylisoquinoline alkaloids and two oxoaporphine alkaloids were obtained in two or three steps from appropriate isoquinolines.

 Please wait while we load your content...
Please wait while we load your content...