Indium-catalyzed oxidative cross-dehydrogenative coupling of chromenes with 1,3-dicarbonyls and aryl rings†
Abstract
An effective indium-catalyzed oxidative cross-dehydrogenative coupling of electronically varied chromenes with 1,3-dicarbonyl compounds and aryl rings has been established. Both the C–H alkylation and arylation proceed smoothly at room temperature to afford diverse α-substituted chromene compounds in up to 91% yields. Besides these two types of C–H components, simple ketones like cyclohexanones also prove to be well tolerated.
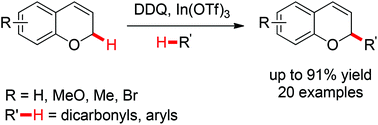

 Please wait while we load your content...
Please wait while we load your content...