Organocatalytic regioselective asymmetric Michael addition of azlactones to o-hydroxy chalcone derivatives†
Abstract
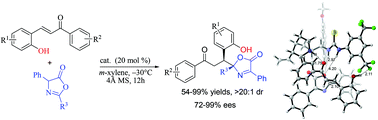
The regioselective and enantioselective Michael addition between azlactones and o-hydroxy chalcone derivatives is reported. Enantiomerically enriched N,O-aminals with two continuous stereogenic centers are exclusively obtained in moderate to good yields with excellent diastereoselectivities and good to excellent enantioselectivities. The experimental results show that an o-hydroxy group on the cinnamenyl motif of chalcone derivatives plays a crucial role at the reaction sites for the regioselective Michael addition. In addition, circular dichroism (CD) spectroscopy and density functional theory (DFT) are used to investigate the absolute configuration of N,O-aminals and the corresponding transition-state structures.

 Please wait while we load your content...
Please wait while we load your content...