Synthesis and complementary self-association of novel lipophilic π-conjugated nucleoside oligomers†
Abstract
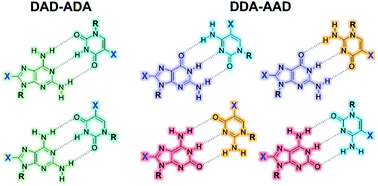
A series of lipophilic nucleosides comprising natural and non-natural bases that are π-conjugated to a short oligophenylene–ethynylene fragment has been synthesized. These bases comprise guanosine, isoguanosine, and 2-aminoadenosine as purine heterocycles, and cytidine, isocytosine and uridine as complementary pyrimidine bases. The hydrogen-bonding dimerization and association processes between complementary bases were also studied by 1H NMR and absorption spectroscopy in order to obtain the relevant association constants.

 Please wait while we load your content...
Please wait while we load your content...