Synthesis of pyrazole containing α-amino acids via a highly regioselective condensation/aza-Michael reaction of β-aryl α,β-unsaturated ketones†
Abstract
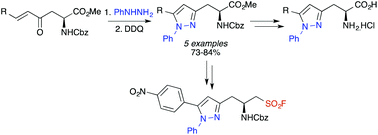
A synthetic approach for the preparation of a new class of highly conjugated unnatural α-amino acids bearing a 5-arylpyrazole side-chain has been developed. Horner–Wadsworth–Emmons reaction of an aspartic acid derived β-keto phosphonate ester with a range of aromatic aldehydes gave β-aryl α,β-unsaturated ketones. Treatment of these with phenyl hydrazine followed by oxidation allowed the regioselective synthesis of pyrazole derived α-amino acids. As well as evaluating the fluorescent properties of the α-amino acids, their synthetic utility was also explored with the preparation of a sulfonyl fluoride derivative, a potential probe for serine proteases.

 Please wait while we load your content...
Please wait while we load your content...