The total synthesis of (−)-cryptocaryol A†
Abstract
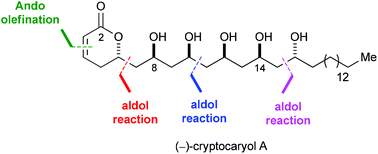
A stereoselective total synthesis of (−)-cryptocaryol A (1) is described. Key features of the 17-step route include the use of three boron-mediated aldol reaction–reduction sequences to control all stereocenters and an Ando modification of the Horner–Wadsworth–Emmons olefination that permitted the installation of the Z double bond of the α-pyrone ring.

 Please wait while we load your content...
Please wait while we load your content...