Indium-catalyzed intramolecular hydroarylation of aryl propargyl ethers†
Abstract
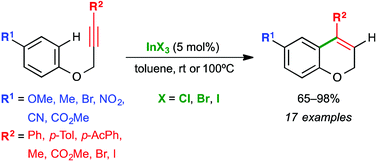
Indium(III) halides catalyze efficiently the intramolecular hydroarylation (IMHA) of aryl propargyl ethers. The reaction proceeds regioselectively with terminal and internal alkynes bearing electron-rich and electron-deficient substituents in the benzenes and alkynes affording only the 6-endo dig cyclization product. Additionally, a sequential indium-catalyzed IMHA and palladium-catalyzed Sonogashira coupling can be performed in one reaction vessel. Experiments with deuterium support a mechanism through electrophilic aromatic substitution.

 Please wait while we load your content...
Please wait while we load your content...