Green and scalable production of colloidal perovskite nanocrystals and transparent sols by a controlled self-collection process†
Abstract
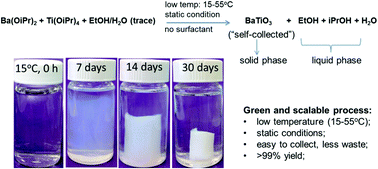
Colloidal perovskite oxide nanocrystals have attracted a great deal of interest owing to the ability to tune physical properties by virtue of the nanoscale, and generate thin film structures under mild chemical conditions, relying on self-assembly or heterogeneous mixing. This is particularly true for ferroelectric/dielectric perovskite oxide materials, for which device applications cover piezoelectrics, MEMs, memory, gate dielectrics and energy storage. The synthesis of complex oxide nanocrystals, however, continues to present issues pertaining to quality, yield, % crystallinity, purity and may also suffer from tedious separation and purification processes, which are disadvantageous to scaling production. We report a simple, green and scalable “self-collection” growth method that produces uniform and aggregate-free colloidal perovskite oxide nanocrystals including BaTiO3 (BT), BaxSr1−xTiO3 (BST) and quaternary oxide BaSrTiHfO3 (BSTH) in high crystallinity and high purity. The synthesis approach is solution processed, based on the sol–gel transformation of metal alkoxides in alcohol solvents with controlled or stoichiometric amounts of water and in the stark absence of surfactants and stabilizers, providing pure colloidal nanocrystals in a remarkably low temperature range (15 °C–55 °C). Under a static condition, the nanoscale hydrolysis of the metal alkoxides accomplishes a complete transformation to fully crystallized single domain perovskite nanocrystals with a passivated surface layer of hydroxyl/alkyl groups, such that the as-synthesized nanocrystals can exist in the form of super-stable and transparent sol, or self-accumulate to form a highly crystalline solid gel monolith of nearly 100% yield for easy separation/purification. The process produces high purity ligand-free nanocrystals excellent dispersibility in polar solvents, with no impurity remaining in the mother solution other than trace alcohol byproducts (such as isopropanol). The afforded stable and transparent suspension/solution can be treated as inks, suitable for printing or spin/spray coating, demonstrating great capabilities of this process for fabrication of high performance dielectric thin films. The simple “self-collection” strategy can be described as green and scalable due to the simplified procedure from synthesis to separation/purification, minimum waste generation, and near room temperature crystallization of nanocrystal products with tunable sizes in extremely high yield and high purity.

 Please wait while we load your content...
Please wait while we load your content...