Formation of hexagonal-molybdenum trioxide (h-MoO3) nanostructures and their pseudocapacitive behavior†
Abstract
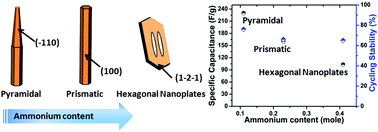
The crystallographic structure and morphology of redox active transition metal oxides have a pronounced effect on their electrochemical properties. In this work, h-MoO3 nanostructures with three distinct morphologies, i.e., pyramidal nanorod, prismatic nanorod and hexagonal nanoplate, were synthesized by a facile solvothermal method. The morphologies of h-MoO3 nanostructures were tailored by a controlled amount of hexamethylenetetramine. An enhanced specific capacitance about 230 F g−1 at an applied current density of 0.25 A g−1 was achieved in h-MoO3 pyramidal nanorods. Electrochemical studies confirmed that the h-MoO3 pyramidal nanorods exhibit superior charge-storage ability. This improved performance can be ascribed to the coexistence of its well exposed crystallographic planes with abundant active sites, i.e., hexagonal window (HW), trigonal cavity (TC) and four-coordinated square window (SW). The mechanism of charge-storage is likely facilitated by the vehicle mechanism of proton transportation due to the availability of the vehicles, i.e., NH4+ and H2O. The promising, distinct and unexploited features of h-MoO3 nanostructures reveal a strong candidate for pseudocapacitive electrode materials.

 Please wait while we load your content...
Please wait while we load your content...