Constructing Fe2O3/TiO2 core–shell photoelectrodes for efficient photoelectrochemical water splitting†
Abstract
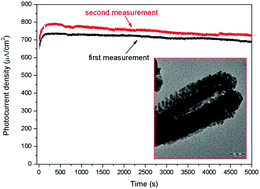
In this study, plasma enhanced chemical vapor deposition (PECVD) was utilized to co-axially modify hydrothermally grown Fe2O3 nanorod arrays by depositing a TiO2 overlayer to create Fe2O3/TiO2 core–shell photoelectrodes. Comprehensive structural (XRD, SEM, TEM) and compositional (XPS) analyses were performed to understand the effects of the TiO2 shell on the PEC activities of the Fe2O3 core. It was revealed that the heterojunction structure formed between TiO2 and Fe2O3, significantly improved the separation efficiency of photo-induced charge carriers and the oxygen evolution kinetics. A maximum photocurrent density of ∼900 μA cm−2 at 0.6 V vs. saturated calomel electrode (SCE) was obtained for the Fe2O3/TiO2 photoelectrodes, which was 5 and 18 times higher when compared to that of hydrothermally synthesized Fe2O3 and PECVD synthesized TiO2 electrodes, respectively. Moreover, the Fe2O3/TiO2 core–shell nanorod arrays displayed superior stability for PEC water splitting. During 5000 s PEC measurements, a steady decrease of the photocurrent was observed, mainly attributed to the evolution of oxygen bubbles adsorbed on the working electrodes. This observation was verified by the complete recovery of the PEC performance demonstrated for a second 5000 s PEC measurement carried out after a brief time interval (10 min) that allowed the electrode surface to regenerate.

 Please wait while we load your content...
Please wait while we load your content...