Toxicity and oxidative stress induced by semiconducting polymer dots in RAW264.7 mouse macrophages
Abstract
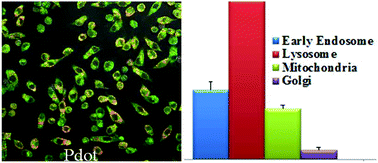
The rapid development and acceptance of PDots for biological applications depends on an in depth understanding of their cytotoxicity. In this paper, we performed a comprehensive study of PDot cytotoxicity at both the gross cell effect level (such as cell viability, proliferation and necrosis) and more subtle effects (such as redox stress) on RAW264.7 cells, a murine macrophage cell line with high relevance to in vivo nanoparticle disposition. The redox stress measurements assessed were inner mitochondrial membrane lipid peroxidation (nonyl-acridine orange, NAO), total thiol level (monobromobimane, MBB), and pyridine nucleotide redox status (NAD(P)H autofluorescence). Because of the extensive work already performed with QDots on nanotoxicity and also because of their comparable size, QDots were chosen as a comparison/reference nanoparticle for this study. The results showed that PDots exhibit cytotoxic effects to a much lesser degree than their inorganic analogue (QDots) and are much brighter, allowing for much lower concentrations to be used in various biological applications. In addition, at lower dose levels (2.5 nM to 10 nM) PDot treatment resulted in higher total thiol level than those found with QDots. At higher dose levels (20 nM to 40 nM) QDots caused significantly higher thiol levels in RAW264.7 cells, than was seen with PDots, suggesting that QDots elicit compensation to oxidative stress by upregulating GSH synthesis. At the higher concentrations of QDots, NAD(P)H levels showed an initial depletion, then repletion to a level that was greater than vehicle controls. PDots showed a similar trend but this was not statistically significant. Because PDots elicit less oxidative stress and cytotoxicity at low concentrations than QDots, and because they exhibit superior fluorescence at these low concentrations, PDots are predicted to have enhanced utility in biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...