One-dimensional cadmium hydroxide nanowires towards electrochemical supercapacitor
Abstract
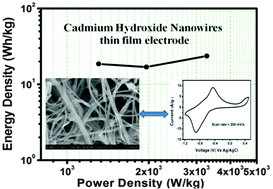
Cadmium hydroxide [Cd(OH)2] nanowires have been successfully synthesized by a simple chemical bath deposition method (CBD) on a stainless-steel (SS) substrate. By scanning electron microscopy (SEM), the surface architecture displays the formation of bundles of high-surface-area nanowires (NWs), which are beneficial for supercapacitor applications. Cd(OH)2 NWs on SS has been tested as an electrode material for supercapacitor applications via electrochemical studies by cyclic voltammetry, charge–discharge and electrochemical impedance spectroscopy techniques in an aqueous electrolyte. Electrochemical data confirm that Cd(OH)2 NWs thin-film electrode exhibits supercapacitor behavior, having a yield of 267 F g−1 specific capacitance at 5 mV s−1 scan rate with excellent cycling life (86% capacitance retention over 1000 cycles). A Cd(OH)2/Cd(OH)2 symmetric supercapacitor device provides a maximum energy density of 11.09 W h kg−1 and a power density of 799 W kg−1 at a current density of 0.84 A g−1. The present work demonstrates that Cd(OH)2 NWs thin film is a promising, low-cost alternative material prepared by a simple, binder-free chemical bath deposition method (CBD) for supercapacitor applications.

 Please wait while we load your content...
Please wait while we load your content...