A versatile method of epoxide formation with the support of peroxy ionic liquids
Abstract
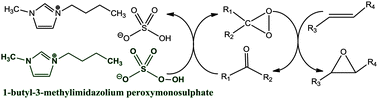
The application of the peroxy ionic liquid, 1-butyl-3-methylimidazolium peroxymonosulphate, as an oxidation agent and a solvent for the synthesis of epoxides was described. The 2.5-molar excess of the peroxy ionic liquid to olefin was applied. The reaction system consisted of 1,1,1-trifluoroacetone as an oxirane precursor, which was used with the molar ratio of 1 : 3 relative to olefin and water solution of NaHCO3. Under these conditions the epoxidation of 4-bromocinnamic acid led to the epoxide formation at the ambient temperature in 30 minutes. Dioxiranes, generated from the peroxy ionic liquid and 1,1,1-trifluoroacetone, demonstrated encouraging potential for the epoxidation of a variety of other olefins: styrene, limonene, stilbene, linalyl acetate and a complex steroid molecule with high yields of final epoxides from 65–98%.

 Please wait while we load your content...
Please wait while we load your content...