Structure and ionization of sulfuric acid in water
Abstract
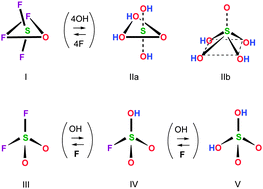
Newly recorded Raman spectra of aqueous sulfuric acid provide scattering shifts of very high sensitivity hence signal-to-noise ratios, at a broad concentration range, up to 17 M. I interpret the spectra as (a) providing no evidence for incomplete H2SO4 ionization at low concentration, (b) reflecting only one dissociation event below ∼5 M, (c) indicating a gradual ion association at ∼5–12.5 M, and (d) exhibiting further structural changes of the sulfate above 12.5 M. The analysis of the Raman shifts supports the postulated presence in solution of the para-bisulfate anion HSO53− as a sole sulfate ion up to ∼5 M, as concluded before (D. Fraenkel, J. Phys. Chem. B, 2012, 116, 11662; D. Fraenkel, J. Phys. Chem. B, 2012, 116, 11678; D. Fraenkel, J. Chem. Thermodyn., 2014, 78, 215), an ion association producing para-sulfuric acid, H4SO5 between 5 and 12.5 M, and a dehydration of H4SO5 to H2SO4 above 12.5 M. These conclusions are rationalized and corroborated by (1) a correlation of the Raman spectra with well-known physicochemical properties of aqueous sulfuric acid, and (2) structural analogies between the proposed para-sulfates and related compounds and anions.

 Please wait while we load your content...
Please wait while we load your content...