Toward white electroluminescence by ruthenium quinoxaline light emitting diodes†
Abstract
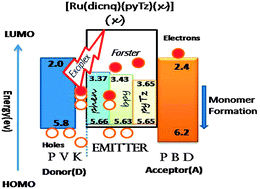
Ancillary ligand substitution in the new series [Ru(dicnq)(pyTz)(X)]+ {dicnq = 6,7-dicyanodipyrido[2,2-d:2′,3′-f]quinoxaline and X = 2,2-bipyridine (bpy), 1,10-phenanthroline (phen), 2-pyridine tetrazole (pyTz)} proves to be an effective way to create white electroluminescence with the Commission Internationale d'Eclairage Chromaticity Coordinates x = 0.40 and y = 0.39. These complexes were characterized by UV-visible and FT-IR spectroscopy, 1H-NMR, CHN and Mass spectroscopy analysis. The absorption and emission spectra are consistent with low-lying MLCT excited states, which are typical of Ru(II) polypyridyl complexes. All the complexes exhibiting reversible metal-centered oxidation processes undergo a one-electron oxidation within the potential range +1.30 vs. SCE. Upon reduction, each compound undergoes several reversible or quasi reversible ligand-centered reduction processes (−1.65 V to −2.40 V). It was found that white light emission can be obtained from the device ITO/PEDOT:PSS/PVK/ruthenium complex/PBD/Al by combining Forster emission from Ru(dicnq) and Exciplex emission at the PVK/Ru(dicnq) interface, although the CIE chromaticity coordinates gradually change with applied voltages. The highest luminous efficiency and luminance and lowest turn-on voltage values are obtained from the 8 wt% Ru(dicnq)(pyTz)(bpy) doped device as 1.78 cd A−1 and 1030 cd m−2 at 16 V and 5.2 V, respectively. As an important result, the incorporation of pyTz as the ancillary ligand into [Ru(dicnq)] complexes was found to be the most beneficial ancillary substitution in terms of creating the white electroluminescence in ruthenium quinoxaline complexes.

 Please wait while we load your content...
Please wait while we load your content...