Synthesis, characterization and antioxidant activity of organoselenium and organotellurium compound derivatives of chrysin†
Abstract
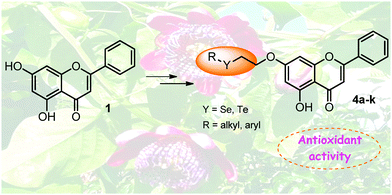
Herein we describe the results on the synthesis and the evaluation of the antioxidant activity of several organochalcogen-containing chrysin derivatives (Se and Te). The semi-synthetic compounds were easily synthesized in good to excellent yields by the reaction of 7-(2-bromoethoxy)-chrysin with nucleophilic organoselenium and organotellurium species. The antioxidant properties of Se- and Te-containing chrysin derivatives were evaluated by three different in vitro assays, the 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging activities and ferric ion reducing antioxidant power (FRAP). Compounds 4i (which contains the butyltellurium moiety) and 4j (which contains a 4-phenyltellurium moiety) exhibited higher activities than chrysin and the selenium analogues, with compound 4i being the most potent antioxidant.

 Please wait while we load your content...
Please wait while we load your content...