One pot domino reaction accessing γ-nitroesters: synthesis of GABA derivatives†
Abstract
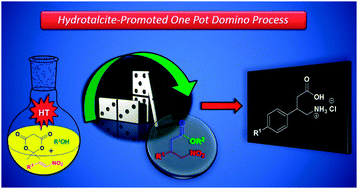
Michael addition of 1,3-dicarbonyl compounds to nitrostyrenes is efficiently promoted by hydrotalcite [Mg–Al] to afford the respective γ-nitrodicarbonyl adducts. Differently, the addition of Meldrum's acid leads to a direct access of γ-nitroesters through a one pot domino process. GABA derivatives (+/−)-phenibut and (+/−)-baclofen were readily synthesized from the respective nitro adducts.

 Please wait while we load your content...
Please wait while we load your content...