Theoretical investigation on a series of novel S,S-dioxide diarylethenes with abnormal photochromic properties and design of new dyads
Abstract
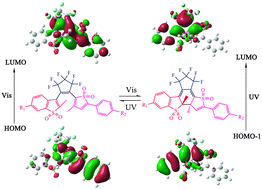
Density functional theory (DFT) calculations are utilized to analyze a novel S,S-dioxide diarylethene of which, contrary to general ones, ring-closing happens under visible light (436 nm) and ring-opening arises in ultraviolet light (365 nm). The asymmetrical structure and the oxidized S atoms alter the continuity and switch method of the conjugation system between the two isomers. The role and position of the substituent affects the intramolecular electron transfer during reaction and results in changes in conversion ratio and quantum yield. Based on the diarylethene, we designed new dyads in which ring-closing and -opening of the two diarylethene parts in one molecule proceed simultaneously under a single wavelength. The photocyclization on one section of the molecule does not limit but facilitate the photocycloreversion on the other section.

 Please wait while we load your content...
Please wait while we load your content...