Electrostatic complementarity in pseudoreceptor modeling based on drug molecule crystal structures: the case of loxistatin acid (E64c)†
Abstract
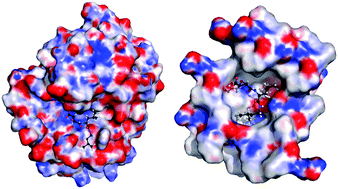
After a long history of use as a prototype cysteine protease inhibitor, the crystal structure of loxistatin acid (E64c) is finally determined experimentally using intense synchrotron radiation, providing insight into how the inherent electronic nature of this protease inhibitor molecule determines its biochemical activity. Based on the striking similarity of its intermolecular interactions with those observed in a biological environment, the electrostatic potential of crystalline E64c is used to map the characteristics of a pseudo-enzyme pocket.

 Please wait while we load your content...
Please wait while we load your content...