Synthesis of high surface area mixed metal oxide from the NiMgAl LDH precursor for nitro-aldol condensation reaction†
Abstract
We have investigated the effect of divalent metal cations on the structural and catalytic activity of MgAl-layered double hydroxide (LDH), by partially substituting Mg2+ with M2+ (M2+ = Ni2+ or Co2+) keeping the metal to aluminium ratio (M2+Mg/Al3+) = 3 and Mg2+/M2+ = 1. The LDH precursors were prepared by a coprecipitation method. The products were finally calcined at 450 °C for 4 h to obtain mixed metal oxides. The catalysts were characterized by powder X-ray diffraction (PXRD), Fourier transform infra-red spectroscopy (FTIR), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and Brunauer–Emmett–Teller (BET) surface area techniques. The catalysts were then tested for nitro-aldol condensation reaction under different reaction conditions. The mixed metal oxide obtained by the calcination of the NiMgAl LDH precursor was found to be a more efficient catalyst for nitro-aldol condensation under solvent free conditions at room temperature due to its high BET-surface area and pore volume.
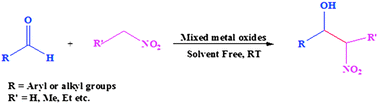

 Please wait while we load your content...
Please wait while we load your content...