Molecular electrostatic potential dependent selectivity of hydrogen bonding†
Abstract
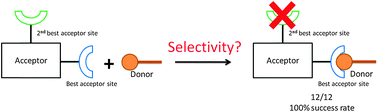
A series of co-crystallizations between four biimidazole based compounds (1,1′-bis(pyridin-4-ylmethyl)-2,2′-biimidazole A1, 1,1′-bis(pyridin-3-ylmethyl)-2,2′-biimidazole A2, 1,1′-bis(pyridin-2-ylmethyl)-2,2′-biimidazole A3 and 1,1′-dibenzyl-2,2′-biimidazole A4) and nine symmetric aliphatic di-acids were carried out in order to determine if a ranking based on calculated molecular electrostatic potential surfaces (MEPS) can be used to effectively assign selectivity in hydrogen-bond based intermolecular interactions. Acceptors A1–A3 are asymmetric ditopic acceptors where two types of nitrogen atoms (pyridine and imidazole) are present, while A4 is a control molecule with only one type of acceptor to rule out any influence of steric factors in the observed structures. 28 of 36 experiments (78% yield) produced a co-crystal, as indicated by relevant changes in the corresponding infrared spectra. Twelve crystal structures of co-crystals of A1–A3 were obtained and every single one displayed primary OH⋯N hydrogens bonds that could be rationalized using the ranking based upon electrostatic potential maps. One crystal structure of A4 was obtained the result of which indicate that the patterns of behaviour established in this family of compounds is not a result of sterics or crystal packing; a simple electrostatic view of hydrogen-bond strength provides a reliable tool for predicting the most likely interactions in a competitive system.

 Please wait while we load your content...
Please wait while we load your content...