Influence of the synthesis route on the formation of 12R/10H-polytypes and their magnetic properties within the Ba(Ce,Mn)O3 family
Abstract
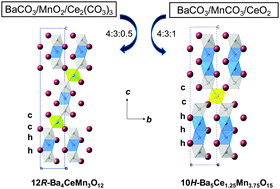
The influence of the Mn precursor on the formation of polytypes was studied within the Ba–Ce–Mn–O system. Minor changes in the mean oxidation state of Mn cations guide the reaction route through different pathways to obtain the iso-stoichiometric 12R-Ba4CeMn4O12−δ or 10H-Ba5Ce1.25Mn3.75O15−δ compounds. This behaviour was controlled using MnO2 or MnCO3 as starting chemical precursors. The understanding of such tendencies allowed the synthesis of the new 10H-Ba5Ce0.83Mn4.17O15−δ composition through a two-step reaction route. This compound crystallizes in a hexagonal symmetry with space group P63/mmc (No. 194) and cell parameters a = b = 5.77650(6) Å and c = 23.8590(4) Å. Its main characteristic is the presence of pure [Mn4O15] oligomers, establishing a difference with respect to the already known 10H-Ba5Ce1.25Mn3.75O15 polytype for which the principal feature was a mixed metal occupancy within [Ce0.25Mn3.75O15] tetramers. This difference generates strong antiferromagnetic (AFM) exchanges, contrary to the metamagnetic-like transition observed in 10H-Ba5Ce1.25Mn3.75O15. It is concluded that full Mn occupancy in [Mn3O12] or [Mn4O15] sub-units induces a robust AFM character.

 Please wait while we load your content...
Please wait while we load your content...