Carbazole–corrole and carbazole–prophyrin dyads: synthesis, fluorescence and electrochemical studies†
Abstract
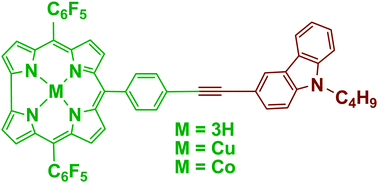
Donor–acceptor type carbazole–corrole and carbazole–porphyrin dyads 4–9 were synthesized and characterized. Corrole and porphyrin containing one meso-p-iodophenyl group were coupled to N-butyl-3-ethynyl-carbazole by a modified Sonogashira reaction. All the dyads were characterized by HRMS mass, NMR, UV-vis absorption, fluorescence and electrochemical techniques. Fluorescence studies of dyads 4, 7 and 8 indicated energy transfer from the donor carbazole moiety to the corresponding acceptor corrole/porphyrin moiety (up to 80%). The electron-releasing N-butyl-carbazole group affects the electronic properties of the corrole and porphyrin macrocycles in the dyads 4–9. Electrochemical studies revealed that dyads 4 and 5 are difficult to reduce compared to their corresponding monomers, as reflected by the cathodic shift in their reduction potentials. Meanwhile, dyads 6–9 exhibited an anodic shift in their reduction potentials, suggesting that they are easy to reduce compared to their corresponding monomers.

 Please wait while we load your content...
Please wait while we load your content...