Ratiometric fluorescent pH probes based on aggregation-induced emission-active salicylaldehyde azines†
Abstract
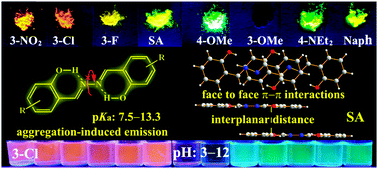
A series of luminescent salicylaldehyde azines (SAs) containing different electron-accepting substituents (–NO2, –F, and –Cl), electron-donating substituents (–OMe and –NEt2), and a π-extended system (naphthalene ring) are prepared for the application of fluorescent pH probes. These SAs inheriting the aggregation-induced emission (AIE) features display strong blue, green, and red fluorescence with large Stokes shifts in water and solid medium. Combining the advantages of AIE and the chemical reactivity of phenol towards OH−/H+, most of the SAs can be used as ratiometric fluorescent pH probes with a broad pH range (2–14) in water and solid medium (test paper). Moreover, the inherent relationship between their chemical structures and AIE properties/pKa values (7.5–13.3) is studied, which provides unequivocal insights into the design of AIE-active dyes and their applications.

 Please wait while we load your content...
Please wait while we load your content...