A microchannel device tailored to laser axotomy and long-term microelectrode array electrophysiology of functional regeneration†‡
Abstract
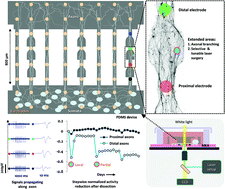
We designed a miniaturized and thin polydimethylsiloxane (PDMS) microchannel device compatible with commercial microelectrode array (MEA) chips. It was optimized for selective axonal ablation by laser microdissection (LMD) to investigate the electrophysiological and morphological responses to a focal injury in distinct network compartments over 45 days in vitro (45 DIV). Low-density cortical or hippocampal networks (<3500 neurons per device) were cultured in quasi-closed somal chambers. Their axons were selectively filtered through neurite cavities and guided into the PDMS microchannels aligned over the recording electrodes. The device geometries amplified extracellularly recorded signals in the somal reservoir and the axonal microchannels to detectable levels. Locally extended areas along the microchannel, so-called working stations, forced axonal bundles to branch out and thereby allowed for their repeatable and controllable local, partial or complete dissections. Proximal and distal changes in the activity and morphology of the dissected axons were monitored and compared to those of their parent networks and of intact axons in the control microchannels. Microscopy images confirmed progressive anterograde degeneration of distal axonal segments over four weeks after surgery. Dissection on cortical and hippocampal axons revealed different cell type- and age-dependent network responses. At 17 DIV, network activity increased in both the somal and proximal microchannel compartments of the dissected hippocampal or cortical axons. At later days (24 DIV), the hippocampal networks were more susceptible to axonal injury. While their activity decreased, that in the cortical cultures actually increased. Subsequent partial dissections of the same axonal bundles led to a stepwise activity reduction in the distal hippocampal or cortical axonal fragments. We anticipate that the MEA-PDMS microchannel device for the combined morphological and electrophysiological study of axonal de- and regeneration can be easily merged with other experimental paradigms like molecular or pharmacological screening studies.

 Please wait while we load your content...
Please wait while we load your content...