Abstract
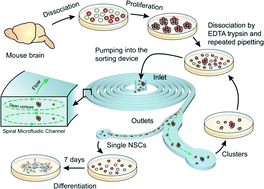
Sphere forming assays are routinely used for in vitro propagation and differentiation of stem cells. Because the stem cell clusters can become heterogeneous and polyclonal, they must first be dissociated into a single cell suspension for further clonal analysis or differentiation studies. The dissociated population is marred by the presence of doublets, triplets and semi-cleaved/intact clusters which makes identification and further analysis of differentiation pathways difficult. In this work, we use inertial microfluidics to separate the single cells and clusters in a population of chemically dissociated neurospheres. In contrast to previous microfluidic sorting technologies which operated at high flow rates, we implement the spiral microfluidic channel in a novel focusing regime that occurs at lower flow rates. In this regime, the curvature-induced Dean's force focuses the smaller, single cells towards the inner wall and the larger clusters towards the center. We further demonstrate that sorting in this low flow rate (and hence low shear stress) regime yields a high percentage (>90%) of viable cells and preserves multipotency by differentiating the sorted neural stem cell population into neurons and astrocytes. The modularity of the device allows easy integration with other lab-on-a-chip devices for upstream mechanical dissociation and downstream high-throughput clonal analysis, localized electroporation and sampling. Although demonstrated in the case of the neurosphere assay, the method is equally applicable to other sphere forming assays.

 Please wait while we load your content...
Please wait while we load your content...