Copper-catalyzed hydroxylation of aryl halides: efficient synthesis of phenols, alkyl aryl ethers and benzofuran derivatives in neat water†
Abstract
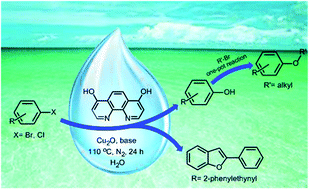
A thorough study of environmentally friendly hydroxylation of aryl halides is presented. The best protocol consists of hydroxylation of different aryl bromides and electron-deficient aryl chlorides by water solution of tetrabutylammonium hydroxide catalyzed by Cu2O/4,7-dihydroxy-1,10-phenanthroline. Various phenol derivatives can be obtained in excellent selectivity and great functional group tolerance. This methodology also provides a direct pathway for the formation of alkyl aryl ethers and benzofuran derivatives in a one-pot tandem reaction.

 Please wait while we load your content...
Please wait while we load your content...