Selective terminal C–C scission of C5-carbohydrates†
Abstract
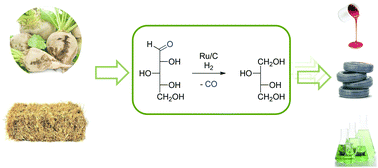
The selective catalytic production of C4-tetritols (erythritol and threitol) from C5-sugars is an attractive route for the conversion of non-digestible sugars to C4-building blocks from agro residues. Here we show that an unprecedented high selectivity of 20–25% C4-tertritols can be achieved under mild conditions (138 °C, 6 bar H2, and 24 h) in the aqueous conversion of xylose over a 5 wt% Ru/C catalyst. A mechanistic study revealed that the dominant reaction mechanism for C5-sugar conversion involves a formal decarbonylation step leading to the initial formation of the desired C4-tetritols. Subsequently the formed C4-tetritols undergo further terminal C–C scissions to glycerol and ethylene glycol. Remarkably, potentially competing reactions like internal C–C chain scission (fragmentation) or hydrodeoxygenation (HDO) do not occur to any significant extent under the applied conditions.

 Please wait while we load your content...
Please wait while we load your content...