Multifunctional pure and Eu3+ doped β-Ag2MoO4: photoluminescence, energy transfer dynamics and defect induced properties†
Abstract
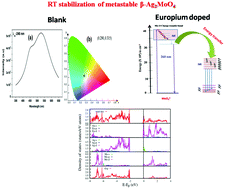
Pure and Eu3+ doped β-Ag2MoO4 were synthesized using a co-precipitation method at room temperature. The as prepared compounds were characterized systematically using X-ray diffraction (XRD), photoluminescence (PL) spectroscopy, cyclic voltammetry (CV) and positron annihilation lifetime spectroscopy (PALS). It is observed that pure β-Ag2MoO4 gives blue (445 nm) and green (550 nm) emission when irradiated with UV light. The origin of the green band was qualitatively explained from density functional theory (DFT) calculations using a suitable distortion model. It was observed that on doping europium ions, efficient energy transfer from molybdate to europium takes place. The excitation spectrum depicting f–f transitions (particularly 395 nm and 465 nm peaks) is much more intense than the CTB showing that Eu3+ ions can be effectively excited by near UV-light. Based on DFT calculations it is proposed that due to the occurrence of Eu3+ d-states in the conduction band (CB) as well as the strong contribution of Eu3+ d-states to the impurity level present in the vicinity of the Fermi level, the host (β-Ag2MoO4) to dopant (Eu3+) energy transfer is preferable. β-Ag2MoO4 is also explored as a potential candidate for electrocatalysis of the oxygen reduction reaction (ORR). It was observed that the doping of europium ions in β-Ag2MoO4 enhances the electrocatalytic activity toward the ORR. The presence of a large concentration of cation vacancies and large surface defects as suggested by positron annihilation lifetime spectroscopy (PALS) seem to be aiding the ORR.

 Please wait while we load your content...
Please wait while we load your content...