Counter-ligand control of the electronic structure in dinuclear copper-tetrakisguanidine complexes†
Abstract
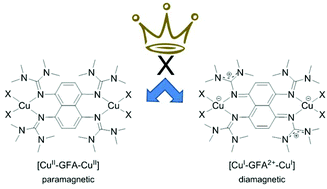
The redox-active GFA (Guanidino-Functionalized Aromatic compound) 1,4,5,8-tetrakis(tetramethylguanidino)-naphthalene (6) is used to synthesize new dinuclear copper complexes of the formula [6(CuX2)2] with different electronic structures. With X = OAc, a dinuclear CuII complex of the neutral GFA is obtained (electronic structure [CuII-GFA-CuII], two unpaired electrons), and with X = Br a diamagnetic dinuclear CuI complex of the dicationic GFA (electronic structure [CuI-GFA2+-CuI], closed-shell singlet state). The different electronic structures lead to significant differences in the optical, structural and magnetic properties of the complexes. Furthermore, the complex [6(CuI)2]2+ (electronic structure [CuI-GFA2+-CuI], closed-shell singlet state) is synthesized by reaction of 62+ with two equivalents of CuI. Slow decomposition of this complex in solution leads to the fluorescent dye 2,7-bis(dimethylamino)-1,3,6,8-tetraazapyrene. In an improved synthesis of this tetraazapyrene, 6 is reacted with CuBr in the presence of dioxygen. Quantum chemical calculations show that the addition of counter-ligands to the trigonal planar CuI atoms of [6(CuI)2]2+ favors or disfavors one of the electronic structures, depending on the nature of the counter-ligand.

 Please wait while we load your content...
Please wait while we load your content...