Synthesis, structure and reactivity of a donor-stabilised silylene with a bulky bidentate benzamidinato ligand†
Abstract
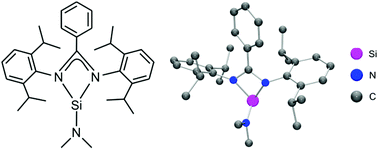
The novel donor-stabilised silylene 5 was prepared in a four-step synthesis, starting from bis(2,6-diisopropylphenyl)carbodiimide (Dipp–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Dipp), and its reactivity was studied in a series of oxidative addition reactions and a nucleophilic substitution reaction. The three-coordinate silicon(II) complex 5 contains the bulky bidentate amidinato ligand Dipp–NC(Ph)N–Dipp− and a dimethylamido ligand. Treatment of 5 with N2O afforded the dinuclear five-coordinate silicon(IV) complex 6 (SiO2N3 skeletons), and the reaction with S8 yielded the dinuclear four-coordinate silicon(IV) complex 7 (SiS2N2 skeletons). Treatment of 5 with Se and Te afforded the respective four-coordinate silicon(IV) complexes 8 (SiSeN3 skeleton) and 9 (SiTeN3 skeleton), which contain an Si
N–Dipp), and its reactivity was studied in a series of oxidative addition reactions and a nucleophilic substitution reaction. The three-coordinate silicon(II) complex 5 contains the bulky bidentate amidinato ligand Dipp–NC(Ph)N–Dipp− and a dimethylamido ligand. Treatment of 5 with N2O afforded the dinuclear five-coordinate silicon(IV) complex 6 (SiO2N3 skeletons), and the reaction with S8 yielded the dinuclear four-coordinate silicon(IV) complex 7 (SiS2N2 skeletons). Treatment of 5 with Se and Te afforded the respective four-coordinate silicon(IV) complexes 8 (SiSeN3 skeleton) and 9 (SiTeN3 skeleton), which contain an Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se and Si
Se and Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Te double bond, respectively. The reaction of 5 with the silyl azide Me3SiN3 yielded the four-coordinate silicon(IV) complex 10 (SiN4 skeleton) with an Si
Te double bond, respectively. The reaction of 5 with the silyl azide Me3SiN3 yielded the four-coordinate silicon(IV) complex 10 (SiN4 skeleton) with an Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond, whereas the reaction with the alkyl azide PhSCH2N3 gave the four-coordinate silicon(IV) complex 11 (SiSN3 skeleton), the first silicon(IV) complex with an unsubstituted methyleneamido ligand. The reaction of 5 with [Fe(CO)5] afforded the four-coordinate silicon(II) complex 12 (SiFeN3 skeleton) with an Si–Fe bond. Compounds 5–12 (and the precursors 14 and 15 (five-coordinate silicon(IV) complexes with an SiCl3N2 and SiCl2N3 skeleton, respectively) in the synthesis of 5) were characterised by elemental analyses, crystal structure analyses and multinuclear NMR spectroscopic studies in the solid state and in solution.
N double bond, whereas the reaction with the alkyl azide PhSCH2N3 gave the four-coordinate silicon(IV) complex 11 (SiSN3 skeleton), the first silicon(IV) complex with an unsubstituted methyleneamido ligand. The reaction of 5 with [Fe(CO)5] afforded the four-coordinate silicon(II) complex 12 (SiFeN3 skeleton) with an Si–Fe bond. Compounds 5–12 (and the precursors 14 and 15 (five-coordinate silicon(IV) complexes with an SiCl3N2 and SiCl2N3 skeleton, respectively) in the synthesis of 5) were characterised by elemental analyses, crystal structure analyses and multinuclear NMR spectroscopic studies in the solid state and in solution.

 Please wait while we load your content...
Please wait while we load your content...