Dual mode signaling responses of a rhodamine based probe and its immobilization onto a silica gel surface for specific mercury ion detection†
Abstract
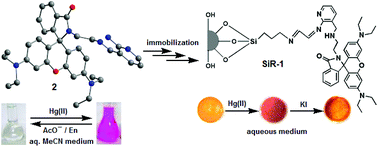
A 3-aminomethyl-(2-amino-1-pyridyl) coupled amino-ethyl-rhodamine-B based probe (2) exhibited simultaneous chromogenic and fluorogenic dual mode signaling responses in the presence of Hg(II) ions only among all the metal ions investigated in an organic aqueous medium. The spiro-cyclic rhodamine signaling subunit undergoes complexation induced structural transformation to result in absorption and fluorescence modulation. Its complexation induced signaling exhibited reversibility with various contrasting reagents having higher affinity towards Hg(II) ions, such as anions (AcO−) and competing chelating agents (En). It also exhibited Hg(II)-specific photophysical signaling responses when immobilized onto a silica gel surface attached through its amino-ethyl-receptor end, owing to its structure-conformational advantages for effective coordination. The surface modified silica appended with 2 (SiR-1), as evaluated through the FTIR spectral pattern, thermogravimetric analysis, FESEM images, elemental analysis, X-ray diffraction, surface area determination and particle size analysis, also exhibited reversible Hg(II)-specific signaling in its suspension state in an aqueous medium, enhancing the probe's utility for practical applications such as the detection, isolation and extraction of Hg(II) ions in the presence of other competitive metal ions.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...