Abstract
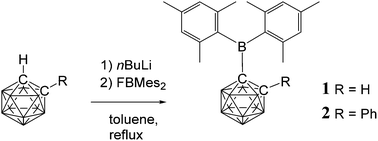
Two C-dimesitylboryl-1,2-dicarba-closo-dodecaboranes, 1-(BMes2)-2-R-1,2-C2B10H10 (1, R = H, 2, R = Ph), were synthesised by lithiation of 1,2-dicarba-closo-dodecaborane and 1-phenyl-1,2-dicarba-closo-dodecaborane, respectively, with n-butyllithium and subsequent reaction with fluorodimesitylborane. These novel compounds were structurally characterised by X-ray crystallography. Compounds 1 and 2 are hydrolysed on prolonged exposure to air to give mesitylene and boronic acids 1-(B(OH)2)-2-R-1,2-C2B10H10 (3, R = H, 4, R = Ph respectively). Addition of fluoride anions to 1 and 2 resulted in boryl-carborane bond cleavage to give dimesitylborinic acid HOBMes2. UV absorption bands at 318–333 nm were observed for 1 and 2 corresponding to local π–π*-transitions within the dimesitylboryl groups while visible emissions at 541–664 nm with Stokes shifts of 11 920–16 170 cm−1 were attributed to intramolecular charge transfer transitions between the mesityl and cluster groups. Compound 2 was shown by cyclic voltammetry to form a stable dianion on reduction. NMR spectra for the dianion [2]2− were recorded from solutions generated by reductions of 2 with alkali metals and compared with NMR spectra from reductions of 1,2-diphenyl-ortho-carborane 5. On the basis of observed and computed 11B NMR shifts, these nido-dianions contain bowl-shaped cluster geometries. The carborane is viewed as the electron-acceptor and the mesityl group is the electron-donor in C-dimesitylboryl-1,2-dicarba-closo-dodecaboranes.
- This article is part of the themed collection: In memory of Professor Kenneth Wade


 Please wait while we load your content...
Please wait while we load your content...