Synthesis of chelating diamido Sn(iv) compounds from oxidation of Sn(ii) and directly from Sn(iv) precursors†
Abstract
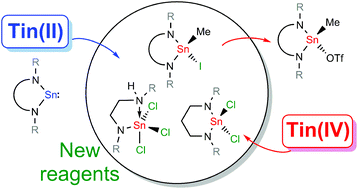
Three dimethyltindiamides containing chelating diamide ligands were synthesised from the reaction of the dilithiated diamine and Me2SnCl2; [SnMe2(L1)] 1 (L1 = κ2-N(Dipp)C2H4N(Dipp)), [SnMe2(L2)] 2 (L2 = κ2-N(Dipp)C3H6N(Dipp)) and [SnMe2(L3)] 3 (L3 = κ2-N(Dipp)SiPh2N(Dipp)), Dipp = 2,6-iPr2C6H3. Reaction of (L2)H2 with SnCl4 and NEt3 led to the formation of the diamidotin dichloride [SnCl2(L2)] 4 whereas reaction of (L1)H2 with SnCl4 and NEt3, or [Sn(L1)] with SnCl4, led to the exclusive formation of the amidotin trichloride [SnCl3{κ2-DippN(H)C2H4N(Dipp)}] 5. Reactions of [Sn(L1)] with sulfur and selenium formed [{Sn(L1)(μ-E)}2] (E = S 10 and Se 11). MeI reacted with N-heterocyclic stannylenes to generate the Sn(IV) addition products [Sn(Me)I(L1)] 12, [Sn(Me)I(L2)] 13, [Sn(Me)I(L3)] 14 and [Sn(Me)I(L4)] 15 (L4 = κ3-N(Dipp)C2H4OC2H4N(Dipp)), and subsequent reaction with AgOTf (OTf = OSO2CF3) generated the corresponding Sn(IV) triflates [Sn(Me)OTf(L1)] 16, [Sn(Me)OTf(L2)] 17 and [Sn(Me)OTf(L4)] 19 with [Sn(Me)OTf(L3)] 18 formed only as a mixture with unidentified by-products. All of the compounds were characterised by single crystal X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...