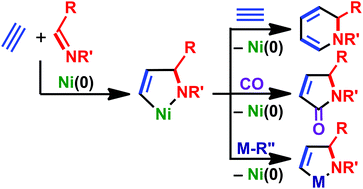
Aza-nickelacycle key intermediate in nickel(0)-catalyzed transformation reactions
Abstract
This Perspective provides an overview of the oxidative cyclization reactions of alkynes and imines with nickel(0) to give five-membered aza-nickelacycles. These reactions could be a key step in multicomponent coupling and cycloaddition reactions to afford nitrogen-containing organic compounds.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...